When was the last time you thought about how the body digests food? If you’re ‘normal’, the last time you probably gave it any more energy than a passing thought was back in seventh grade health class. Back then you probably learned that saliva mixes with you food when you chew it (if you chew it) and then hydrochloric acid in the stomach breaks it all down into a slurry that then passes through the intestines where your body absorbs the nutrients. After that, well, I think we all know what we end up with!
Yet, it’s a bit more interesting than that. That is, if you’re not ‘normal’.
So, today, we’re going to take a closer look at this process. The closer look will specifically be regarding what the body absorbs rather than how the body absorbs. There is a distinction here that is fairly subtle, yet profoundly simple. If that distinction is understood, the process of how the body absorbs becomes a simple mechanical process for what the body absorbs is what’s important.
Sugar Molecules
To start with, everyone knows that food gives us energy. You eat an apple, and we can say you consumed roughly 70 calories of energy. To get to those calories, the digestive process has to break down the plant cell structures to release the (roughly) 20 grams of carbohydrates. Those twenty grams of carbohydrates (a fairly complex collection of molecules) are then broken down into simple sugars (smaller molecules) that can be absorbed into the body.
A simple sugar can look like the following combination of glucose and fructose.

Once in the body, the sugar molecules, that can be of many different forms (molecular shapes), are then dispersed to the cells where they can perform cellular respiration to extract the energy. Cellular respiration is a generally a chemical oxidation process where when sugar is combined with oxygen to release the energy (light), carbon dioxide and water.
In this case, the body absorbed sugar molecules derived from some carbohydrates. Carbohydrates come in many different forms: Monosaccharides, Disaccharides Oligosaccharides and polysaccharides. The following diagram shows a simple combination of one type of saccharide.
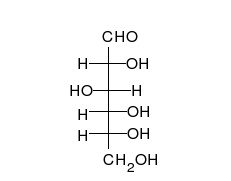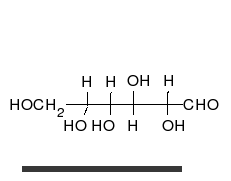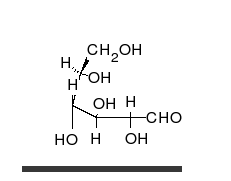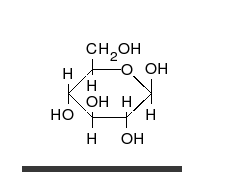
Protein Molecules
Amino acids are the molecules that when put together create protein molecules. Amino acids are considered the basic building blocks for the molecules that our bodies build and use. As we all know, during the digestive process, the body breaks down proteins (longer strains of amino acids) into their basic form in order to assimilate them into the body. When it does, these amino acids can be used to not only produce new molecules, but be used to create energy. From Wikipedia:
When taken up into the human body from the diet, the 22 standard amino acids are either used to synthesize proteins and other biomolecules, or are oxidized to urea and carbon dioxide as a source of energy.
The basic common component in a protein molecule can be seen from this Wikipedia diagram.
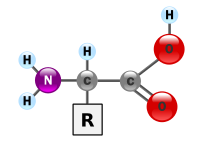
If you take a look at another diagram they offer at Wikipedia, you’ll notice that amino acids very based on the “R” position of the above diagram. They state:
An alpha-amino acid has the generic formula H2NCHRCOOH, where R is an organic substituent;[1] the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (the α–carbon).
Which is supported by their diagram:

As it turns out, there are essential and nonessential amino acids. The general context in which the essential term applies here is in humans. The cool part about amino acids is that they can easily be combined by what’s called the Peptide bond and that they can be broken down to produce energy through the urea cycle.
The most interesting thing about this class of molecules is that scientists are discovering different forms on a regular basis. It’s as if the key thing that these molecules have in common is the ‘connector’. What hangs off the connector as the organic substituent can have many different forms and, one must assume, different functions for each form.
As it turns out, someone has done a little math giving a rough idea regarding the number of variations.
One cheminformatics study[6] identified 849,574 unique substituents up to 12 non-hydrogen atoms large and containing only C,H,N,O,S,P,Se and the halogens in a set of 3,043,941 molecules. Fifty common substituents are found in only 1% of this set, and 438 in 0.1%. 64% of the substituents are unique to just one molecule. The top 5 consists of the phenyl, chlorine, methoxy, hydroxyl, and ethyl substituent. The total number of organic substituents in organic chemistry is estimated at 3.1 million, creating a total of 6.7×1023 molecules.
These things come across as tinker-toys or Legos and there is a huge number of them.
When you think about the absorption of these molecules in the human, if the body picks them up via their connector, there could be virtually anything hanging off of it as the unique substituent. Even though science has isolated some really basic ones, the set that is available shows that there are a lot more that are yet to be discovered, or, explored to see how they function in the body.
Vitamin Molecules
As it turns out, vitamins are also molecules that the body absorbs and many of them seem to take on forms that are similar to amino acids but they are not considered amino acids. One example that I came across is Vitamin B5 (also known as Pantothenic acid). It looks like this:
http://en.wikipedia.org/wiki/File:Pantothenic_acid_structure.svg
Vitamin B1 (Thiamine) looks like this:

Vitamin b2 (Riboflavin) looks like this:
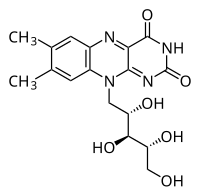
This list goes on and on, but the key thing here is that these are molecules that are slightly different then amino acids that are key components for different functions within the body.
And, as with the amino acid molecules, new vitamin molecules are named fairly regularly. Any time scientists can specifically outline a process in which the catalyst is discovered, we get to learn about it as a new vitamin.
Mineral Molecules
The area that I find most interesting is the mineral molecules. We all know that minerals serve key roles in our lives, but what most people don’t understand is that there is a difference between bio-available and non-bio-available forms of minerals.
Even though common sidewall in houses is made of Gypsum (calcium sulfate dehydrate), breaking off a piece and eating it doesn’t have the same positive affect that consuming calcium from a green leave might provide. In fact, minerals in their raw form can often be detrimental to the body.
Upon researching this article, I came across a molecule that carries iron; Heme. One form of it looks like this:
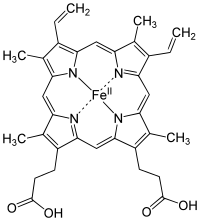
This molecule plays a key part in the oxidation process. It works in the hemoglobin to carry oxygen around to the cells so they can use that oxygen to generate energy.
Magnesium is another mineral that finds an organic transporter. That is what we know of as the chlorophyll molecules. There are a number of different types of chlorophyll molecules. This is just one of them:
http://en.wikipedia.org/wiki/File:Chlorophyll_c1.svg
Organic life also finds that cobalt is also needed and forms a molecule that’s known as Cobalamin. This is the key part of vitamin B12. It looks like this:
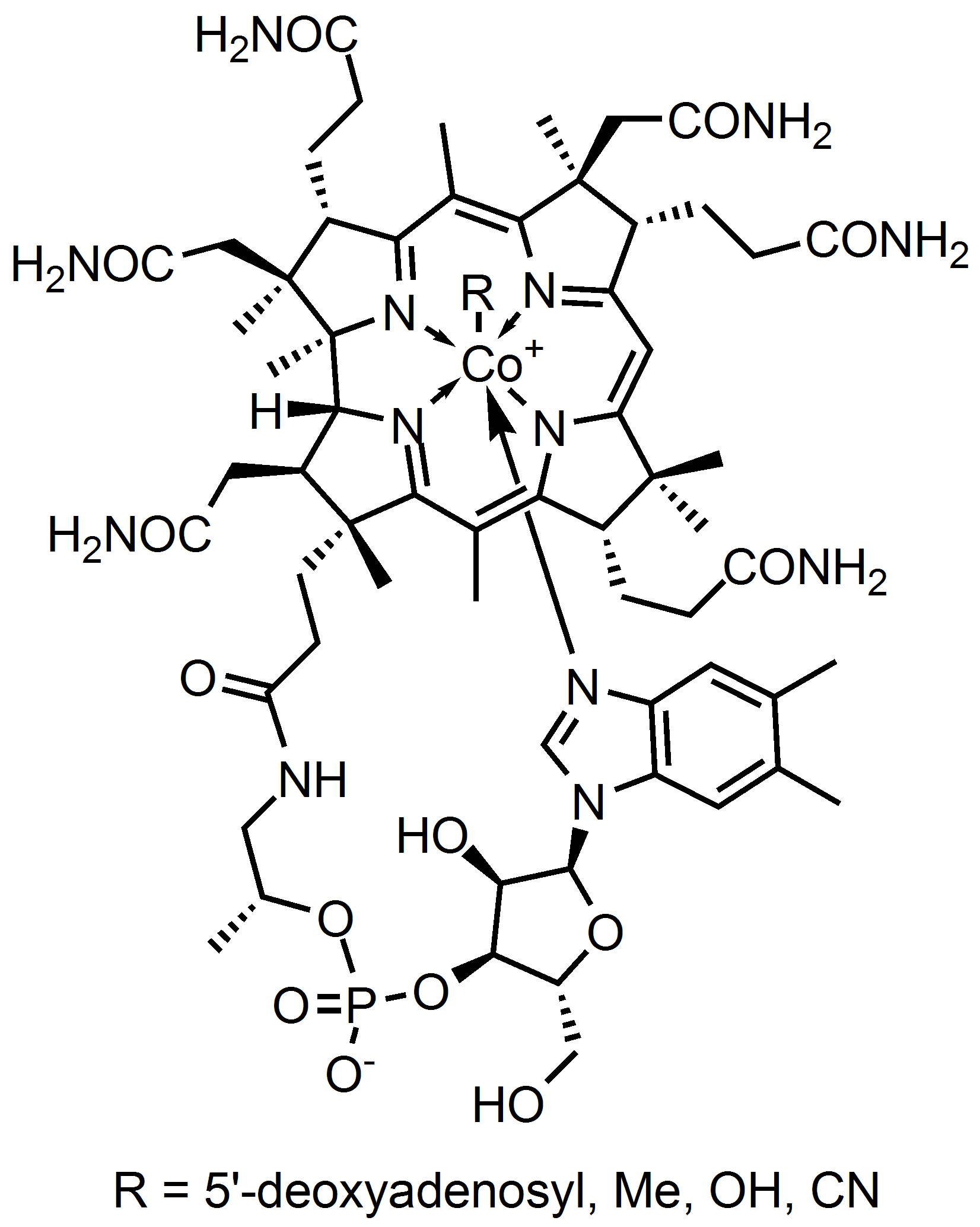
There are more examples, but I’ll get you get the idea. The key takeaway in these molecules is that we find a single atom of the mineral combined with the other organic matter. We don’t find combined minerals (like so often found in the ground) and we don’t find attached clusters – like two iron atoms.
Other common molecules
Another well known molecule is beta-Carotene. Wikipedia states:
β-Carotene is an organic compound and classified as a terpenoid.
As they state, this molecule is of the class terpenoid. If we follow that link we find that:
The terpenoids (pronounced /ˈtɜrpɨnɔɪd/ TUR-pə-noyd), sometimes called isoprenoids, are a large and diverse class of naturally-occurring organic chemicals similar to terpenes, derived from five-carbon isoprene units assembled and modified in thousands of ways. Most are multicyclic structures that differ from one another not only in functional groups but also in their basic carbon skeletons. These lipids can be found in all classes of living things, and are the largest group of natural products.
Plant terpenoids are used extensively for their aromatic qualities. They play a role in traditional herbal remedies and are under investigation for antibacterial, antineoplastic, and other pharmaceutical functions. Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, and the color of yellow flowers. Well-known terpenoids include citral, menthol, camphor, Salvinorin A in the plant Salvia divinorum, and the cannabinoids found in Cannabis.
The steroids and sterols in animals are biologically produced from terpenoid precursors. Sometimes terpenoids are added to proteins, e.g., to enhance their attachment to the cell membrane; this is known as isoprenylation.
Which opens up another class of molecules that the body absorbs in subtle ways. Yet, everyone knows that if you eat too much carrot juice you’ll start to turn orange.
Similar to this compound is Lycopene, the latest bus word from tomatoes. Its molecular structure looks like this:
![]()
Hormone Molecules
Here is another class of molecules that the human body can absorb. The Wikipedia shows a list of common human hormones that looks to be pretty long. In this list, we find amine-, peptide- and steroid- versions. Here is Testosterone and Thyroxine:


In the case of Thyroxine, the molecular formula shows that there are 4 iodine atoms. Testosterone simple contains carbon, hydrogen and oxygen.
Other natural molecules
There are other naturally occurring molecules that are small enough to be absorbed in the body, the first one that comes to mind is caffeine.

Or, maybe, the active ingredients in chocolate; theobromine and phenethylamine.
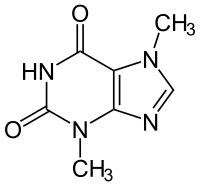

Or what about the active ingredient in pot, Tetrahydrocannabinol (THC).

Summary
I’m sure there are more common collections of molecules that I’ve overlooked here, but the main idea is that when we ingest food, the molecules that exist in the food get absorbed into the body. And, as we’ve all discovered, one type of plant may have a specialized molecule that acts like a trigger to produce some cascading sequence of events that may be witnessed consciously.
For instance, if you eat pot, the active ingredient, THC, is absorbed by the body and the affect is consciously noticeable. Likewise, if you were to consume Viagra, (molecule found here) its affects can clearly be noticed by the body. Caffeine and alcohol are two other really simple examples. In each case, the body didn’t actively produce these molecules, but when they are introduced they trigger responses that may, or may not be desired.
In the standard case, it appears that root molecules are highly absorbable even if it is considered a toxin to the body (alcohol). Mineral compounds are also known to be absorbed even if they are not in a bio-available molecule. Drugs, compounds that the body has never seen before also find their way into the system.
What is still unclear to me is does the body have the ability to absorb amino acid chains? Or, are their larger structures that are seen as ‘safe’ and combine to be brought into the digestive cells only later to find out that it was not useful?
Conclusion
I hope you find this research thought provoking for it helped me gain strength regarding the fact that when humans eat, the body absorbs molecules and it’s not very discerning regarding what it takes in. If you drop a poisonous molecule into your mouth and swallow, chances are pretty high that your body will process that molecule.
This article has also made me think differently about another article that I posted Why people should not eat animals. In that article I stated that the body not only absorbs these ‘simple’ molecules, but it may possible absorb strings of molecules or proteins. Even though I can find specific examples showing that the body absorbs strings of proteins, I have discovered that the body will absorb just about everything else. May there be situations where the protein strings are short enough to make it into the system? I will keep looking for I still believe there may be common situations.
If you have any questions, let me know. Unless, of course, you want to stay ‘normal’.

